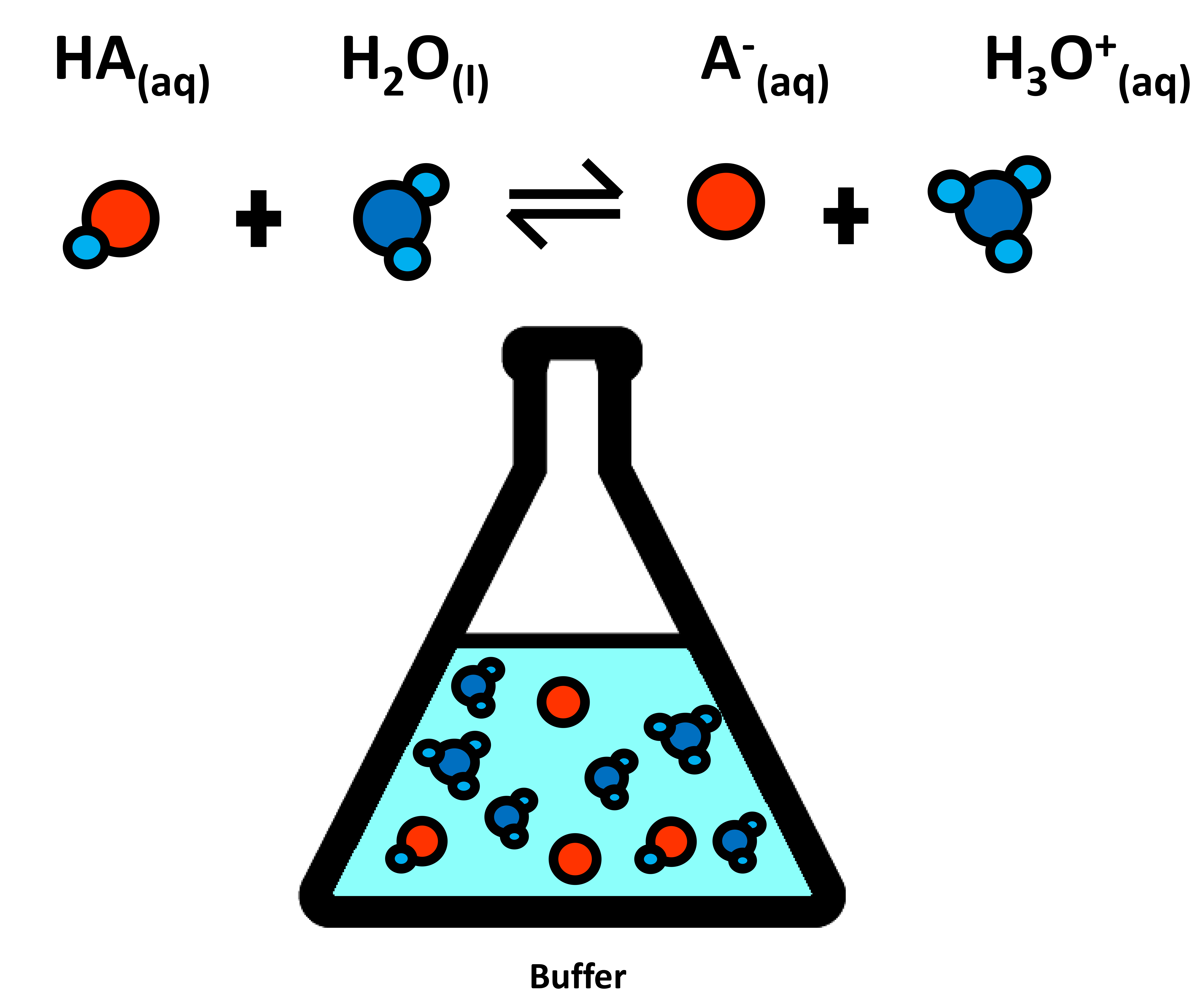
Buffer With Strong Acid . a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. buffers made from weak bases and salts of weak bases act similarly. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Because hc 2 h 3 o 2 is a weak acid, it. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). For example, in a buffer containing nh 3.
from goldbio.com
a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers made from weak bases and salts of weak bases act similarly. Because hc 2 h 3 o 2 is a weak acid, it. a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). For example, in a buffer containing nh 3.
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio
Buffer With Strong Acid a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. Because hc 2 h 3 o 2 is a weak acid, it. a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. For example, in a buffer containing nh 3. a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers made from weak bases and salts of weak bases act similarly.
From www.youtube.com
Strong Acid + Buffer YouTube Buffer With Strong Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. For example, in a buffer containing nh 3. . Buffer With Strong Acid.
From www.youtube.com
AP chemistry Adding Strong Acid to Buffer problem YouTube Buffer With Strong Acid For example, in a buffer containing nh 3. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer solutions resist a change in ph. Buffer With Strong Acid.
From www.numerade.com
SOLVEDWhat species are in the buffer region of a weak acidstrong base titration? How are they Buffer With Strong Acid the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. buffers made from weak bases and salts of weak bases act similarly. a. Buffer With Strong Acid.
From www.youtube.com
Adding strong acid to a buffer (Ch 2, 27) YouTube Buffer With Strong Acid buffers made from weak bases and salts of weak bases act similarly. Because hc 2 h 3 o 2 is a weak acid, it. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer solution is one in which the ph of the solution is. Buffer With Strong Acid.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube Buffer With Strong Acid Because hc 2 h 3 o 2 is a weak acid, it. buffers made from weak bases and salts of weak bases act similarly. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). buffer solutions resist a change in ph when small amounts of a strong acid or. Buffer With Strong Acid.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration and Buffer Region and Buffer With Strong Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. For example, in a buffer containing nh 3. Because hc 2 h 3 o 2 is a weak acid, it. a buffer solution is one in which the ph of the solution is resistant. Buffer With Strong Acid.
From www.brainkart.com
Buffers Buffer With Strong Acid buffers made from weak bases and salts of weak bases act similarly. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a buffer. Buffer With Strong Acid.
From study.com
AcidBase Buffers Calculating the pH of a Buffered Solution Video & Lesson Transcript Buffer With Strong Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers made from weak bases and salts of weak bases act similarly. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. . Buffer With Strong Acid.
From www.youtube.com
The Change in pH with the Addition of a Strong Acid to a Buffer YouTube Buffer With Strong Acid buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. For example, in a buffer containing nh 3. Because hc 2 h 3. Buffer With Strong Acid.
From courses.lumenlearning.com
Buffers Chemistry for Majors Buffer With Strong Acid Because hc 2 h 3 o 2 is a weak acid, it. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). buffers made from weak bases and salts of weak bases act similarly. For example, in a buffer containing nh 3. a mixture of a weak acid and. Buffer With Strong Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 Buffer With Strong Acid For example, in a buffer containing nh 3. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer is a solution that resists changes in its ph. Buffer With Strong Acid.
From www.writework.com
Titration of amino acids WriteWork Buffer With Strong Acid buffers made from weak bases and salts of weak bases act similarly. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. Because hc 2 h 3. Buffer With Strong Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Buffer With Strong Acid buffers made from weak bases and salts of weak bases act similarly. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). Because hc 2. Buffer With Strong Acid.
From www.youtube.com
pH of a buffer after strong acid addition YouTube Buffer With Strong Acid For example, in a buffer containing nh 3. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. buffers made from weak bases and salts of weak bases act. Buffer With Strong Acid.
From exosxgjvz.blob.core.windows.net
Weak Acid Titration Curve Buffer Region at Paula Rivera blog Buffer With Strong Acid a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia. Buffer With Strong Acid.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio Buffer With Strong Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers made from weak bases and salts of weak bases act similarly. the buffer solution in this demo is composed of household vinegar (acetic acid) and ammonia (a weak base). a buffer. Buffer With Strong Acid.
From www.youtube.com
Adding a Strong Acid to a Buffer Solution YouTube Buffer With Strong Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. For example, in a buffer containing nh 3. . Buffer With Strong Acid.
From courses.lumenlearning.com
15.1 Buffers Chemistry Buffer With Strong Acid a buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. a buffer is a solution that resists changes in its ph when small amounts of strong acid or base is added to. Because hc 2 h 3 o 2 is a weak acid,. Buffer With Strong Acid.